|
| Short Contents | Full Contents | Other books NCBI |
|
The Cell  Introduction Introduction  The Chemistry of Cells The Chemistry of Cells
 The Molecular Composition
of Cells The Molecular Composition
of Cells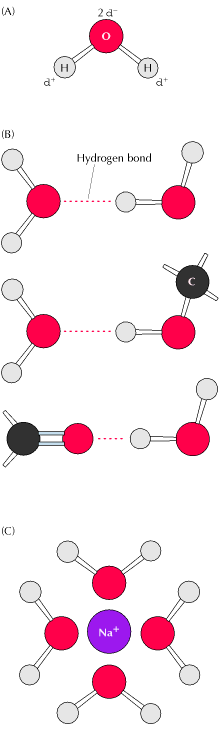 Figure 2.1. Characteristics of water (A) Water is a polar molecule, with a slight negative charge (d-) on the oxygen atom and a slight positive charge (d+) on the hydrogen atoms. Because of this polarity, water molecules can form hydrogen bonds (dashed lines) either with each other or with other polar molecules (B), in addition to interacting with charged ions (C). |